Brand Name Drugs And Generics: How Patents Work
 Generics have been a salvation for Americans who struggle to pay the cost of brand name drugs. Yet while we are all grateful for affordable drugs, people often wonder about the enormous disparity in price between branded and generic drugs.
Generics have been a salvation for Americans who struggle to pay the cost of brand name drugs. Yet while we are all grateful for affordable drugs, people often wonder about the enormous disparity in price between branded and generic drugs.
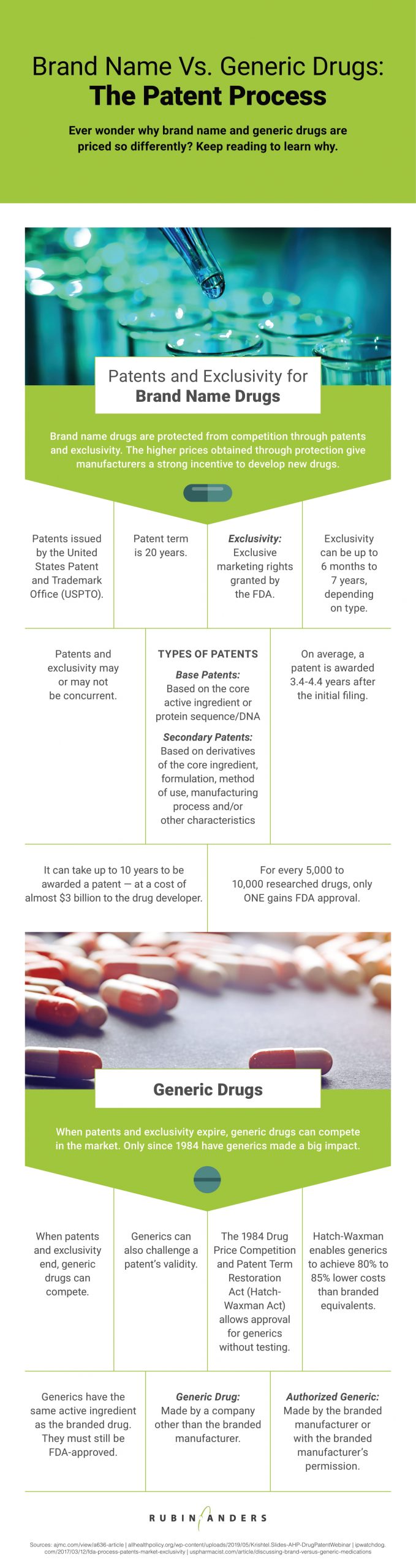
The accompanying resource sheds light on that question. One of the first things you may notice in the resource is that the patent process is long, complex, expensive — and seldom successful. Drug companies spend billions researching new drugs, most of which never hit the pharmacy shelves. Without patent protection — and the ability to charge high prices that goes along with that protection — drug manufacturers would never be able to recoup the investment they make in research and development. Without that R&D, we’d all be much worse off, medically speaking.
Nevertheless, the U.S. government recognized that paying for brand name drugs in perpetuity was a burden few Americans could bear. In 1984, the Hatch-Waxman Act opened the door for generic drugs to compete after a certain length of time, by eliminating the requirement that generics must go through pre-clinical and clinical trials just as the branded products had done. By removing this costly and time-consuming requirement, generics began to enter the market much more quickly, and at greatly reduced prices.
With regard to generics, the resource points out a significant distinction of which readers should certainly be aware if they are taking a generic medication. There are two types of generic drugs: generic drugs and authorized generic drugs.
An authorized generic is manufactured by the company that makes the branded drug and markets it as a generic, or is manufactured by another company with the permission of the original manufacturer.
A non-authorized generic drug is made by a company other than the original manufacturer. Consumers may regard authorized generics as having stronger quality control and/or more consistent availability, which are important considerations especially for drugs that are being used long-term.
For consumers taking branded drugs where no generic is available, the generic alternative might be months away — or decades. A patent is awarded for 20 years, and drug companies extend market protection by obtaining various types of marketing exclusivity rights from the FDA. Naturally, drug companies would like to retain exclusivity as long as possible. However, U.S. law allows competitors to challenge drug patents; if successful, these challenges enable generics to come to market more quickly.
To learn more about branded and generic drugs and the patent process, please continue reading the featured resource below. Courtesy of Rubin Anders